DISCLAIMER
The information and materials accessed through or made available for use on any of our Sites, including, any information about diseases, conditions, treatments, or medicines, are for informational purposes only. The Content is not intended to be and is not a substitute for professional medical advice, diagnosis, or treatment, and your participation on our Sites does not create a healthcare professional-patient relationship. You should consult a doctor or other qualified health care professional regarding any questions you have about your health or before making any decisions related to your health or wellness. Call your doctor or 911 immediately if you think you may have a medical emergency.compose your message
message sent
email sent successfully
Trusted Resources: News & Events
Latest announcements and gatherings
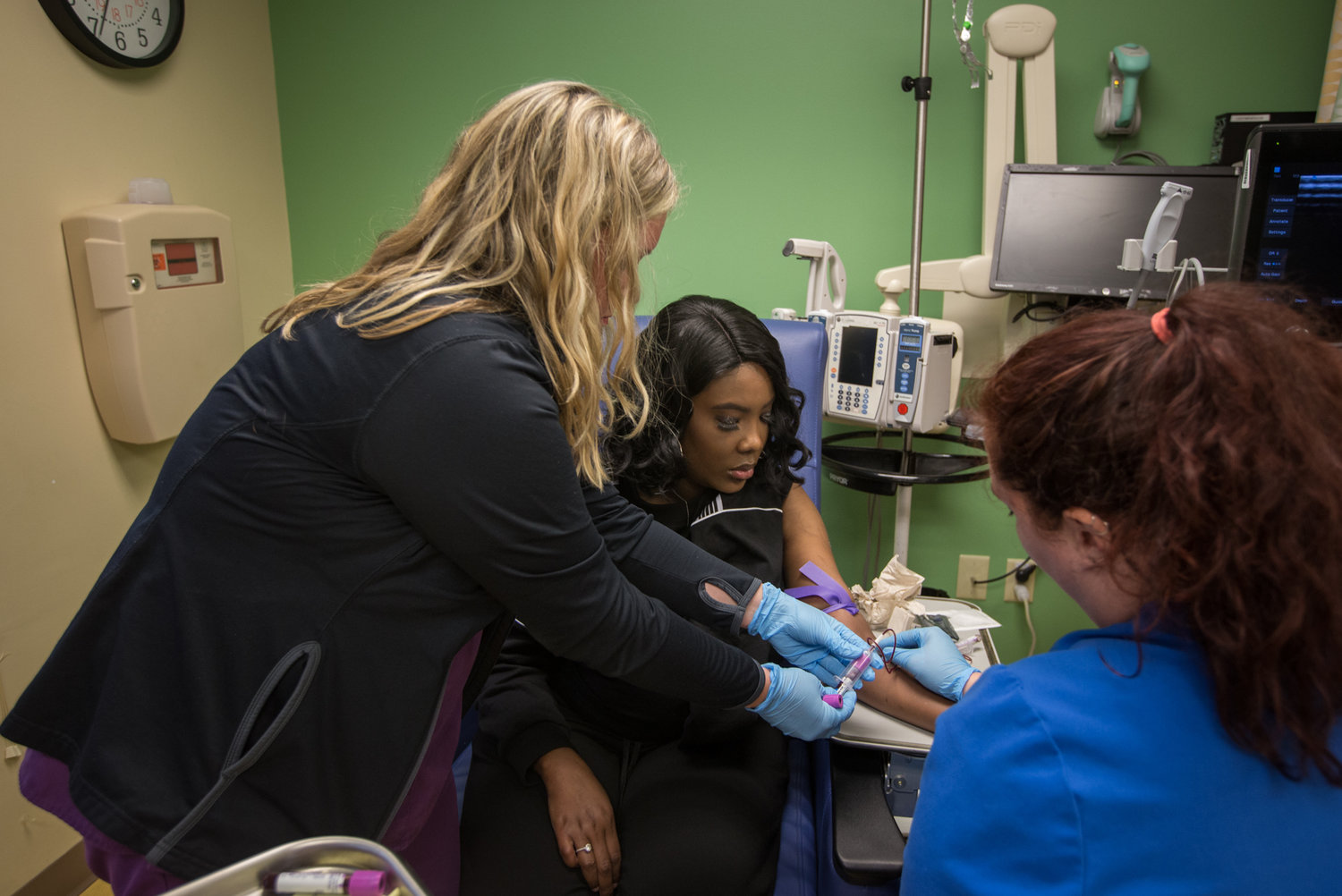
Gene-Edited ‘Supercells’ Make Progress In Fight Against Sickle Cell Disease
Doctors are reporting the first evidence that genetically edited cells could offer a safe way to treat sickle cell disease, a devastating, incurable disorder that afflicts millions of people around the world.
Billions of cells that were genetically modified with the powerful gene-editing technique called CRISPR have started working, as doctors had hoped, inside the body of the first sickle cell patient to receive the experimental treatment, according to highly anticipated data released Tuesday.
The edited cells are producing a crucial protein at levels that have already exceeded what doctors thought would be needed to alleviate the excruciating, life-threatening complications of the genetic blood disorder, the early data show. Moreover, the cells appear to have already started to spare the patient from the agonizing attacks of pain that are the hallmark of the disorder.
“We are very, very excited,” says Dr. Haydar Frangoul of the Sarah Cannon Research Institute in Nashville, Tenn., who is treating the patient. “This preliminary data shows for the first time that gene editing has actually helped a patient with sickle cell disease. This is definitely a huge deal.”
 +myBinder
+myBinderRelated Content
-
people & placesRobert H. Broyles, PhDRobert H. Broyles was founder and presid...
-
people & placesFroedtert Hospital, Sickle Cell ClinicSickle cell disease (SCD) is a genetic d...
-
people & placesSickle Cell Program at UI HealthThe Sickle Cell Program at UI Health is ...
-
people & placesCindy Neunert, MDCindy Neunert, MD is a practicing pediat...
-
education & researchAn analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomesOBJECTIVE: To identify characteristics ...
-
Community CenterOur Healthcare System Abandons Adult Sickle Cell PatientsWhen Janoi Burgess was a child, he thoug...
-
news & eventsThese patients had sickle-cell disease. Experimental therapies might have cured themScientists have long known what causes s...
send a message
To improve your experience on this site, we use cookies. This includes cookies essential for the basic functioning of our website, cookies for analytics purposes, and cookies enabling us to personalize site content. By clicking on 'Accept' or any content on this site, you agree that cookies can be placed. You may adjust your browser's cookie settings to suit your preferences. More Information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.
Support for this site is provided by

This platform is made possible through a partnership with the Sickle Cell Disease Association of America, Inc. (SCDAA) and its member organizations. SCDAA's mission is to advocate for people affected by sickle cell conditions and empower community-based organizations to maximize quality of life and raise public consciousness while advancing the search for a universal cure.




